Magnesium sulfate can be obtained at a drugstore as Epsom salts. The monohydrate is found as the mineral kieserite. What would be the pH of a 500 mL aqueous solution containing 1.62 g kieserite? The 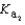 value for sulfuric acid is 1.2 *10-2.
value for sulfuric acid is 1.2 *10-2.
A) 7.00
B) 6.85
C) 7.15
D) 3.55
E) 12.22
Correct Answer:
Verified
Q2: When sodium chloride is added to a
Q22: Acid-base indicators need to have very intense
Q53: Magnesium sulfate can be obtained at a
Q59: Stalactites-the long, icicle-like formations that hang from
Q61: Which of the following compounds would not
Q63: A phosphate buffer solution (25.00 mL sample)
Q94: What is the pH of a 0.15
Q94: The pH of an aqueous sodium fluoride
Q113: The stronger the acid, _
A)the stronger its
Q146: If 500 mL of a solution containing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents