Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 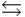 H2PO4- + H3O+
H2PO4- + H3O+ 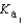 H2PO4- + H2O
H2PO4- + H2O 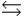 HPO42- + H3O+
HPO42- + H3O+ 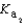 HPO42- + H2O
HPO42- + H2O 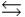 PO43- + H3O+
PO43- + H3O+ 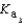 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
A) HPO42- + H2O 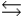 PO43- + H3O+
PO43- + H3O+
B) H3PO4 + H2O 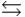 H2PO4- + H3O+
H2PO4- + H3O+
C) PO43- + H2O 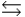 HPO42- + OH-
HPO42- + OH-
D) H2PO4- + H2O 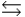 H3PO4 + OH-
H3PO4 + OH-
E) 2H2O 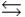 H3O+ + OH-
H3O+ + OH-
Correct Answer:
Verified
Q15: Suppression of the solubility of one ion
Q39: Phosphoric acid is a triprotic acid, ionizing
Q45: What is the hydronium ion concentration of
Q45: Stalactites-the long, icicle-like formations that hang from
Q46: Aqueous solutions of _ are basic.
A) NaF
B)
Q47: What is the pH of a 0.010
Q52: What is the concentration of [OH-] in
Q72: What is the pH of a 0.20
Q87: Which one of the following salts forms
Q148: What is the solubility of barium sulfate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents