The following titration curve is most likely to be associated with 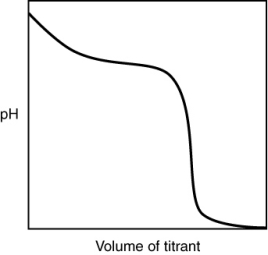
A) the titration of a strong acid with a strong base titrant.
B) the titration of a weak acid with a strong base titrant.
C) the titration of a strong base with a strong acid titrant.
D) the titration of a weak base with a strong acid titrant.
Correct Answer:
Verified
Q3: A solution that contains a weak acid
Q4: Which of the following is not a
Q22: Acid-base indicators need to have very intense
Q39: What are the characteristics of a pH
Q63: A phosphate buffer solution (25.00 mL sample)
Q69: A 0.500 g sample of an unknown
Q72: When an acetic acid solution is titrated
Q87: As the pH decreases, the solubility of
Q129: The most suitable acid-base indicator for a
Q133: Halfway to the equivalence point in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents