At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL. The sharp rise is at 10.0 mL. 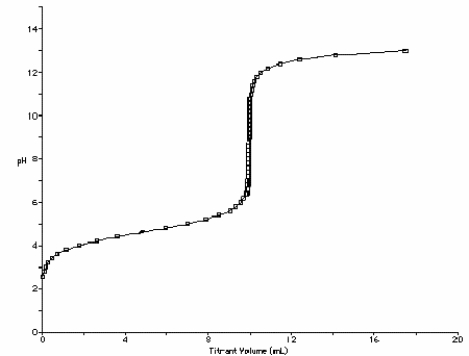
A) 0.0 mL
B) 5.0 mL
C) 9.0 mL
D) 10.0 mL
E) 18.0 mL
Correct Answer:
Verified
Q12: Which combination of solutions is the best
Q29: Glycolic acid, which is a monoprotic acid
Q46: One brand of extra-strength antacid tablets contains
Q101: Write the reaction and equilibrium constant that
Q101: Which of the following would be the
Q131: A mining operation needs to separate silver
Q137: Lead pipes were used at one time
Q139: What is the pH of a solution
Q153: The solubility of PbBr2 is 0.427 g
Q154: Define a Brønsted-Lowry acid and a Brønsted-Lowry
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents