A chemical equilibrium 2 A 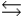 B
B
Has a forward rate constant, kf = 10 M -1s-1, and a reverse rate constant, kr = 5.0 s-1. What is the value of the equilibrium constant for this system?
A) 0.5
B) 2.0
C) 20
D) 0.050
E) 5.0
Correct Answer:
Verified
Q1: The forward rate constant, kf, and reverse
Q2: Which choice A-D includes quantities that
Q4: Write the equilibrium expression for the following
Q5: Consider the equilibrium A + B
Q6: A chemical equilibrium 2 A 
Q8: Identify the equilibrium expression for the following
Q8: For an equilibrium reaction with K =
Q10: The equilibrium constant for the acid ionization
Q11: An equilibrium that strongly favors products has
Q12: Which of A-D is equal in an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents