For the chemical equilibrium aA + bB 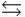 cC, the value of the equilibrium constant, K, is 10. What is the value of the equilibrium constant for the following reaction? cC
cC, the value of the equilibrium constant, K, is 10. What is the value of the equilibrium constant for the following reaction? cC 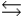 aA + bB
aA + bB
A) 0.10
B) 10
C) 1
D) 100
E) -10
Correct Answer:
Verified
Q20: The equilibrium constant for the formation of
Q22: In equilibrium expressions, the concentrations of pure
Q23: For the chemical equilibrium aA + bB
Q24: Which of the following is the equilibrium
Q27: Write the expression for the reaction quotient
Q29: A series of four equilibrium steps can
Q48: Sulfur dioxide emitted in the burning of
Q60: If the reaction quotient Q has a
Q74: Addition of reactants to a chemical reaction
Q77: Which of the following occurs when reactants
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents