The gas-phase equilibrium of the oxidation of sulfur dioxide, shown here, has an equilibrium constant, Kp, value of 4.17 *102 at 200 C. If a closed vessel was filled with sulfur trioxide and the initial pressure of SO3 was 0.033 atm at 200 C, what would be the final partial pressure of SO3? 2SO2(g) + O2(g) 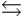 2SO3(g)
2SO3(g)
A) more than 0.033 atm
B) less than 0.033 atm
C) 0.033 atm exactly
D) there is no way to tell
Correct Answer:
Verified
Q42: The sulfide ion, S2-, reacts with water
Q43: Cylinders of NO gas may contain small
Q44: Which of the following relationships are
Q45: What happens to the equilibrium between NO2(g)
Q47: A student determined the equilibrium concentration in
Q48: In the ICE table started for calculating
Q49: In the equation relating equilibrium to
Q53: What is the difference between
Q65: Which of the following must be
Q72: Which of the following is true regarding
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents