The G  of atomic oxygen is 230.1 kJ/mol. Determine the equilibrium constant for the following reaction at standard thermodynamic conditions. O2(g)
of atomic oxygen is 230.1 kJ/mol. Determine the equilibrium constant for the following reaction at standard thermodynamic conditions. O2(g) 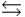 2O(g)
2O(g)
A) 4.7 * 10-41
B) 2.3 *10-61
C) 2.2 * 10-81
D) 2.1 * 1040
E) 4.5 * 1080
Correct Answer:
Verified
Q59: Which of the following occurs when products
Q60: To solve an equation of the form
Q62: A sketch of the free energy for
Q64: If K for a reaction is
Q65: When plotting lnK vs. 1/T, a
Q66: A sketch of the free energy
Q76: Increasing the temperature of an exothermic reaction
Q106: As the temperature of a reaction
Q131: The prediction of linearity in a
Q132: The equilibrium constant for a given reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents