If the rate of formation of ammonia is 0.345 M/s, what is the rate of disappearance of N2? 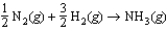
A) 0.173 M/s
B) 0.345 M/s
C) 0.690 M/s
D) 245 M/s
E) 0.518 M/s
Correct Answer:
Verified
Q28: For the rate law Rate = k[A][B]1/2,
Q30: For the rate law Rate = k[A]3/2[B],
Q31: For the rate law Rate = k[A]1/2[B],
Q32: A scientist conducts an experiment to
Q34: A scientist conducts an experiment to
Q34: If the rate of formation of
Q37: If the reaction 3A + B
Q44: In a rate law, the partial orders
Q56: The reaction
A + 2B
Q60: Which of these could be the units
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents