The rate of disappearance of HI in the reaction 2HI(g) I2(g) + H2(g) is shown in the following figure. What is the instantaneous rate of this reaction at t = 5 s? 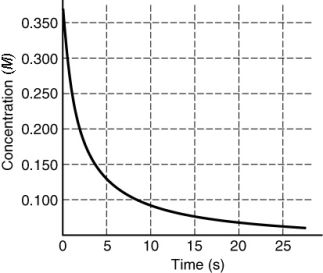
A) 0.2 M/s
B) 0.01 M/s
C) 0.02 M/s
D) 0.100 M/s
E) 0.04 M/s
Correct Answer:
Verified
Q6: NO2 contributes to the "brown haze" associated
Q10: When one chemical is a precursor of
Q12: The greatest NO concentration is observed _
A)in
Q16: For the reaction 2A + 3B
Q20: Which of the following is not a
Q24: HI dissociates to form I2 and
Q25: The difference between an average rate and
Q26: For the rate law Rate = k[A][B]3/2,
Q32: Which of the following does the initial
Q35: If the rate of the reaction:
2O3(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents