The mechanism for the reaction 2H2O2(aq) 2H2O( 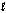 ) + O2(g) in the presence of I-(aq) is proposed to be: Step 1: H2O2(aq) + I-(aq) H2O(
) + O2(g) in the presence of I-(aq) is proposed to be: Step 1: H2O2(aq) + I-(aq) H2O( 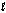 ) + OI-(aq)
) + OI-(aq)
(slow)
Step 2: H2O2(aq) + OI-(aq) H2O( 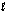 ) + O2(g) + I-(aq)
) + O2(g) + I-(aq)
(fast)
What is the molecularity of the rate-determining step?
A) zeromolecular
B) unimolecular
C) bimolecular
D) termolecular
E) More information is needed to answer this question.
Correct Answer:
Verified
Q80: The following energy profiles for four different
Q82: The reaction of NO gas with
Q83: The mechanism for the reaction 2H2O2(aq)
Q86: A proposed mechanism for the reduction
Q87: Given the following data for the
Q88: The mechanism for the reaction 2H2O2(aq)
Q89: A proposed mechanism for the reduction
Q90: Which of the following reaction pathways shows
Q113: A reaction mechanism consists of a series
Q125: A proposed mechanism for the decomposition
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents