Calculate the lattice energy of sodium fluoride from the following data: Ionization energy of Na: 496 kJ/mol
Electron affinity of F: -328 kJ/mol
Energy to vaporize Na: 108 kJ/mol
F2 bond energy: 160 kJ/mol
Energy change for the reaction:
Na(s) +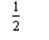 F2(g) NaF(s) ; H = -575 kJ
F2(g) NaF(s) ; H = -575 kJ
A) 931 kJ/mol
B) -931 kJ/mol
C) -1011 kJ/mol
D) 1011 kJ/mol
E) -851 kJ/mol
Correct Answer:
Verified
Q7: Which of the solutions shown here will
Q8: Determine the energy change for the
Q11: Which of the solutions shown here will
Q12: Which of the following will have the
Q21: The vapor pressure of an aqueous solution
Q23: At 25°C, the vapor pressure of pure
Q31: Indicate which aqueous solution has the fastest
Q43: Which listing of ionic compounds is in
Q51: Which of the following is needed to
Q77: Indicate which aqueous solution has the slowest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents