Ion interaction energies are determined by Coulomb's law: 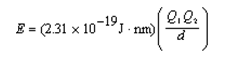 Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
A) -7.36 *10-19 J
B) -7.36 *10-22 J
C) 7.36 *10-19 J
D) 1.74* 10-18 J
E) -7.36 * 10-25 J
Correct Answer:
Verified
Q2: Which of the following will require the
Q5: For a molecule to exhibit dipole-dipole interactions,
Q7: For molecules or atoms with the same
Q10: Arrange the three compounds sodium chloride, magnesium
Q11: Predict which of the following compounds has
Q11: Which of the following requires the smallest
Q15: Which of the following solvents will involve
Q17: Coulomb's law states that the energy of
Q17: The interaction energy of LiF is -1.14
Q27: Which of the following compounds is capable
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents