Which of the following diagrams best shows a set of polar molecules interacting through dipole-dipole interactions?
A) 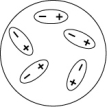
B) 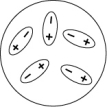
C) 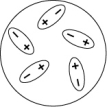
D) 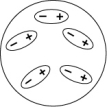
Correct Answer:
Verified
Q1: Ion-dipole forces always require_
A)an ion and a
Q3: Which of the following will have the
Q6: Which of the following polar compounds is
Q9: Based on their boiling points, which of
Q18: Which of the following compounds is capable
Q21: Polarizability refers to _
A)the ease with which
Q25: The solubility of a compound is determined
Q27: Hydrophilic substances _
A)are immiscible in water.
B)are insoluble
Q56: Which of the following pairs of compounds
Q109: Given the van der Waals a constant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents