Which statement about the phase diagram below is not correct? 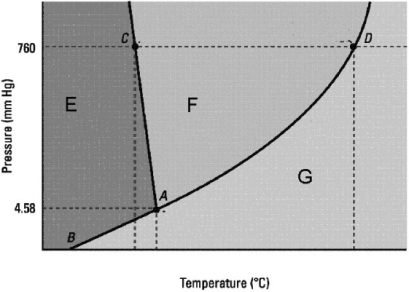
A) The critical point is not shown.
B) D is the normal boiling point.
C) The vapor pressure of the solid is zero below the triple point.
D) The normal melting point is at a lower temperature than the triple point.
E) E, F, and G label in that order the solid, liquid, and gas phases.
Correct Answer:
Verified
Q14: A hydration sphere forms around an ion
Q59: Consider the phase diagram for a substance
Q61: Which one of the following substances would
Q62: The temperature at point b in the
Q65: The relative energies (strengths) of the intermolecular
Q66: Point d in the phase diagram below
Q68: Rank the following compounds in order of
Q69: Which of the substances a-d in the
Q118: Water forms a concave meniscus in a
Q127: The boiling point of HBr is higher
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents