Which of the following shows the orientation of the net dipole for OCS?
A) 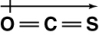
B) 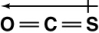
C) 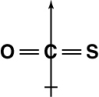
D) 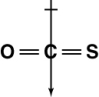
Correct Answer:
Verified
Q30: Which of the following has a central
Q33: What is the molecular geometry of SF4O?
A)pentagonal
B)tetrahedral
C)trigonal
Q40: Which of the following molecules or ions
Q41: What is the hybridization of the oxygen
Q48: Identify the polar molecule.
A)CF4
B)SiH4
C)CHCl3
D)CS2
E)CO2
Q54: What is the hybridization of the central
Q65: Which of the following compounds has the
Q70: Which of the following compounds is the
Q86: What type of hybridization is needed to
Q92: What type of hybridization is needed to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents