Which of these molecules have a dipole moment? 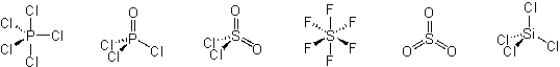
A) PCl5 and SiCl4
B) POCl3, SO2Cl2, and SO3
C) POCl3 and SO2Cl2
D) PCl5, POCl3, SOCl2, SO3, and SiCl4
E) PCl5, SF6, SO3, and SiCl4
Correct Answer:
Verified
Q61: Which of the following statements about bonds
Q67: Boron nitride, BN, is a new high
Q68: According to the molecular orbital energy level
Q77: Which one of the following molecules is
Q103: Which of the following molecules has a
Q111: Use energy levels of diatomic molecules derived
Q113: Predict the bond order of the NO+
Q118: What is the bond order of B2?
A)0
B)1
C)2
D)3
E)1.5
Q126: Which one of the following molecules has
Q140: Which of the following molecules is paramagnetic?
A)Li2
B)C2
C)B2
D)N2
E)F2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents