Based on the following graph, which metal will emit the highest energy photoelectron when a photon with a wavelength of 650 nm is incident on the metal? 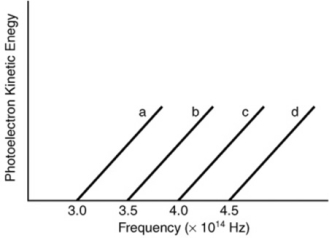
A) a
B) b
C) c
D) d
Correct Answer:
Verified
Q4: The study of light emitted by the
Q17: Which of the following photons has
Q24: Which of the following will lead to
Q29: What is the energy (E, in J)
Q31: If each of the following metals is
Q35: Which of the following photons has
Q36: What is the energy (E, in
Q39: Which of the following sources produces the
Q50: Which of the following metals would be
Q58: What wavelength of light is required to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents