The energy of a one-electron atom is given by E = -(2.18 * 10-18 J) 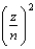 where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
A) Li2+
B) Be3+
C) He+
D) B4+
E) C5+
Correct Answer:
Verified
Q42: The change in energy of a
Q43: What Q43: What is the speed of an argon Q44: Which of the transitions in the hydrogen Q45: Which of the transitions in the following Q47: A shell is defined by _ Q55: Which transition in a hydrogen atom will Q66: De Broglie reasoned that for the electron Q80: In quantum mechanics, an atomic orbital _ Q96: Which of the following is not an![]()
A) the
A)provides
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents