A shell consists of all __________
A) electrons with the same 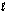 quantum number.
quantum number.
B) orbitals with the same quantum numbers.
C) orbitals with the same principal quantum number.
D) electrons with the same magnetic quantum number.
E) orbitals with the same 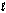 and ml quantum numbers.
and ml quantum numbers.
Correct Answer:
Verified
Q41: How much energy is required to ionize
Q46: Determine the wavelength of the line in
Q52: Recently, buckyballs (C60) became the largest object
Q54: Which of the following electrons will have
Q57: Which of the following objects, all moving
Q61: Which combination of quantum numbers is possible
Q62: Which of the following represents an s
Q65: Subshells are _
A)orbitals with the same principal
Q71: The mathematical description of an electron as
Q78: According to de Broglie, if the circumference
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents