What is the orbital designation for an electron with the quantum numbers n = 3, 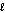 = 2?
= 2?
A) 3s
B) 3d
C) 2p
D) 2f
E) 3e
Correct Answer:
Verified
Q75: Which of the following is a possible
Q76: How many orbitals are possible for the
Q81: Give the names of the three quantum
Q83: A certain shell is known to have
Q106: What is the ground-state electron configuration of
Q108: Which arrangement is correct for increasing atomic
Q114: Which of the following atoms or ions
Q132: What is the minimum energy that a
Q136: Write the electron configuration of Zn2+.
Q164: Arrange the following elements in order of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents