The following diagrams illustrate the flow of energy (q) and work (w) in different processes. Which one is definitely an exothermic process?
A) 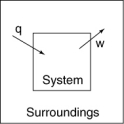
B) 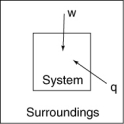
C) 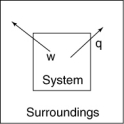
D) 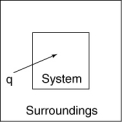
Correct Answer:
Verified
Q4: The kinetic energy associated with the random
Q7: The capacity to do work is a
Q11: In addition to identifying the reactants and
Q12: A balanced reaction equation with a
Q16: Thermochemistry is the study of how _
Q17: Energy that an object has by virtue
Q26: The first law of thermodynamics implies that
Q29: During a(n) _ process, energy is transferred
Q30: Which of the following is an endothermic
Q32: During a(n) _ process, energy is transferred
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents