In terms of the enthalpy of formation, which of the following compounds is the most unstable compared to its elements under standard conditions?
A) KCl(s) , H 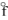 = - 435.9 kJ/mol
= - 435.9 kJ/mol
B) PH3(g) , H 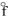 = +5.4 kJ/mol
= +5.4 kJ/mol
C) Fe3O4(s) , H 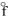 = - 1117.1 kJ/mol
= - 1117.1 kJ/mol
D) NiO(s) , H 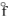 = - 239.7 kJ/mol
= - 239.7 kJ/mol
E) ICl(g) , H 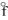 = + 17.8 kJ/mol
= + 17.8 kJ/mol
Correct Answer:
Verified
Q31: If a chemical reaction causes the temperature
Q39: In a steam engine, steam in a
Q41: A 150 g piece of iron (CP
Q42: A 15 g piece of iron (CP
Q43: You have a summer job in a
Q45: Which of the following objects will cool
Q46: The energy content of a Big Mac
Q47: When a 13.0 g sample of NaOH(s)
Q49: You hold a 50 g sphere of
Q86: Indicate which of the following is not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents