Which of the following fuels has the highest fuel value (kJ/g) ?
A) natural gas, CH4(g) ; 16.0 g/mol, Hcomb = -801 kJ/mol
B) ethanol, CH3CH2OH( 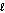 ) ; 46.1 g/mol, Hcomb = -1,368 kJ/mol
) ; 46.1 g/mol, Hcomb = -1,368 kJ/mol
C) heating oil, C16H34( 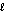 ) , 226 g/mol, Hcomb = -10,800 kJ/mol
) , 226 g/mol, Hcomb = -10,800 kJ/mol
D) coal, C135H96O9NS(l) , 1906 g/mol, Hcomb = - 44,000 kJ/mol
E) wood, C6H12O6(l) , 180 g/mol, DHcomb = -2,540 kJ/mol
Correct Answer:
Verified
Q60: In an experiment, 30.0 g of metal
Q63: Which of the following substances, A-D, will
Q68: How much energy is needed to change
Q69: There is concern that the combustion
Q87: Ethanol (CH3CH2OH) has been suggested as
Q92: Which statement regarding combustion of a sample
Q108: Assume gasoline has an energy density of
Q111: Suppose you eat a one-third pound hamburger
Q118: Fuel density is _
A)the cost of energy
Q118: Lightweight camping stoves typically use a mixture
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents