Identify the base in the following acid-base reaction. PbCO3(s) + H2SO4(aq) PbSO4(s) + CO2(g) + H2O( 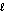 )
)
A) CO32-
B) CO2
C) SO42-
D) H2O
E) H2SO4
Correct Answer:
Verified
Q22: Hydroxyapatite, [Ca5(PO4)3(OH)], the major component of
Q22: In a demonstration of strong electrolytes, weak
Q23: Which picture best represents an atomic-level view
Q25: Identify the acid in the following
Q28: Which one of the following statements regarding
Q30: Which contains more solute particles: a 0.10
Q48: In a demonstration of strong electrolytes, weak
Q49: What is the molar concentration of sodium
Q56: In a demonstration of strong electrolytes, weak
Q60: Chalk contains calcium carbonate. What would be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents