What would be the products in the ionic equation for the following reaction? 2HBr(aq) + 2KOH(aq) products
I. K+(aq) and Br-(aq) ;
II. KH(aq) ;
III. H2O( 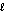 ) ;
) ;
IV. KBr(s) ; V. BrOH(aq) ; VI. KBr(aq)
A) I and III
B) III and IV
C) III and VI
D) II, III, and V
E) II and V
Correct Answer:
Verified
Q30: Which contains more solute particles: a 0.10
Q36: Which one of the following statements regarding
Q37: Calcium hydroxide is slightly soluble in water.
Q38: Two phosphoric acid molecules can combine to
Q43: If the molar concentration of sodium sulfate
Q48: In a demonstration of strong electrolytes, weak
Q50: If 50.0 mL of a 0.10 M
Q51: In its reaction with water, ammonia (NH3)
Q56: In a demonstration of strong electrolytes, weak
Q61: Ammonia (NH3) is a weak base that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents