Calcium hydride (CaH2) is so reactive with water that it can be used to remove traces of water from solvents other than water. If 24.6 g of calcium hydride is added to a large volume of solvent that contains 14.0 g of water, which of these remains, water or calcium hydride, after the reaction is complete? The reaction is: CaH2(s) + 2H2O( 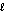 ) Ca(OH) 2(s) + 2H2(g)
) Ca(OH) 2(s) + 2H2(g)
A) CaH2 will be left over.
B) Water will be left over.
C) Both will be completely consumed.
D) There is no way to tell.
Correct Answer:
Verified
Q73: What is the mass in amu of
Q80: Dialuminum hexachloride, Al2Cl6, is an inexpensive
Q87: Socrates was killed by a dose of
Q89: Chemical analysis of an organic compound found
Q107: Write a definition of the term molar
Q107: How many atoms are there in one
Q109: What mass of phosphoric acid (H3PO4, 98.00
Q122: Write the balanced reaction equation for the
Q130: Yeast converts glucose into ethanol and
Q139: Write a definition of the phrase "stoichiometry
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents