A bottle containing 1665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The emergency spill kit contained a full 2.0 kg bottle of sodium carbonate (105.99 g/mol) . Is this enough sodium carbonate to neutralize the acid according to the following reaction? H2SO4(aq) + Na2CO3(s) Na2SO4(aq) + CO2(g) + H2O( 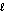 )
)
A) Yes, there is about 50% more than what is needed.
B) Yes, there is exactly enough sodium carbonate, but no excess.
C) No, there is not enough sodium carbonate, but the amount is only about 10% too small.
D) No, there is not nearly enough sodium carbonate.
E) Yes, but there is only about 10% excess.
Correct Answer:
Verified
Q63: A careless student who was instructed
Q69: A mass of 11.60 g of phosphoric
Q71: You are given a liquid sample that
Q73: What is the mass in amu of
Q86: A reaction vessel contains equal masses of
Q93: Allicin, a potent antibacterial compound, is formed
Q95: Combustion analysis of an organic compound to
Q104: U.S. Lime & Minerals is a company
Q104: Household bleach contains 5.0% sodium hypochlorite
Q118: Ammonia, NH3, is an important industrial chemical.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents