According to the phase diagram given, which of the following statements is INCORRECT? 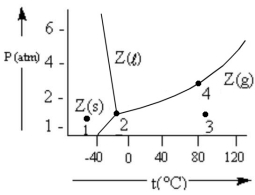
A) At the temperature and pressure of point 2, substance Z exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 3, substance Z exists as a one-phase gaseous system.
C) If the Z(s) = Z(l) = Z(g) system is maintained at the temperature of point 2 while pressure is decreased, more Z will vaporize.
D) If liquid Z is maintained at the pressure of point 4 while the temperature is decreased to 30°C, the liquid will vaporize.
E) The existence of liquid Z at -50°C and 2 atm represents the metastable condition of "supercooling."
Correct Answer:
Verified
Q27: A liquid is in equilibrium with its
Q33: Which of the following is true assuming
Q34: What is the difference between "normal boiling
Q35: Choose the INCORRECT statement.
A) In a network
Q35: Which of the following describe the critical
Q37: Vaporization occurs more readily with:
A) increased temperature,
Q38: The phenomenon of supercooling refers to the
Q41: Three types of holes of the cubic
Q43: The property of a liquid that measures
Q59: The property that causes water to have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents