According to the phase diagram given, which of the following statements is INCORRECT? 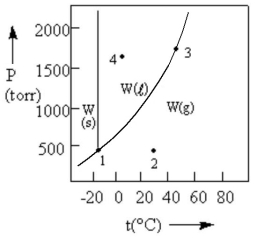
A) At the temperature and pressure of point 1, W exists as a three-phase equilibrium system.
B) At the temperature of point 2, a pressure of 500 torr will cause W to liquefy.
C) If the system is maintained at the temperature of point 3 while pressure is decreased, more W will vaporize.
D) If W is maintained at the pressure of point 4 while the temperature is increased to 80°C, the liquid will vaporize.
E) The existence of liquid W at -40°C and 500 torr represents the metastable condition of "supercooling."
Correct Answer:
Verified
Q22: According to the phase diagram given, which
Q22: Under which of the following conditions will
Q24: Which of the following statements about viscosity
Q26: The enthalpy of fusion is:
A) The quantity
Q29: A passage of substance directly from the
Q31: When a liquid is in dynamic equilibrium
Q31: A liquid has a normal boiling point
Q33: When a liquid is in equilibrium with
Q36: Which of the following keeps the rate
Q37: The phenomenon in which a steel needle
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents