For the molecule 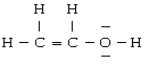
A) the geometry about O is linear
B) the hybridization on O is sp
C) O is not hybridized
D) both carbons are sp2 hybridized
E) there are two π bonds between the two carbons
Correct Answer:
Verified
Q29: Find the correct statements about the bonding
Q30: Choose the INCORRECT statement about SnCl2.
A) The
Q32: For the molecule Q32: π bonds: Q33: Which combination of hybrid orbital descriptions and Q35: The double covalent bond between two carbon Q36: Three of the molecular shapes which a Q37: For BeCl2, the dipole moment of the Q38: Choose the INCORRECT statement about HCN. Q38: Choose the INCORRECT statement about PCl5.![]()
A)are the only kind of bonds
A)There are
A) There
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents