One resonance structure of N2O is 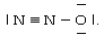 The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
A) four sp3 orbitals
B) three sp2 orbitals and a p orbital
C) two sp2 orbitals and two sp orbitals
D) two sp orbitals and two p orbitals
E) one sp orbital and two p orbitals
Correct Answer:
Verified
Q63: If the wave functions describing the 2s
Q78: The "electron-sea" model for bonding in metals,
Q80: How would replacing one of benzene's C
Q81: What is the hybridization of the Xe
Q82: The structure of aspirin is given below.
Q84: What would be the bond order of
Q85: Which is the correct molecular orbital diagram
Q86: The hybridization on Xe in XeF4 is
Q87: What would be the hybridization of the
Q88: The three molecular shapes an sp3 hybridized
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents