Calculate the enthalpy change for the following reaction at 25°C. The value of ΔH°f in kJ/mol is given below each species: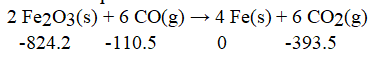
A) -49.6 kJ
B) 541 kJ
C) -1380 kJ
D) -3350 kJ
E) -24.8 kJ
Correct Answer:
Verified
Q41: Calculate the amount of heat absorbed by
Q44: Calculate the quantity of heat,in kJ,required to
Q45: An example of a fossil fuel is:
A)the
Q49: The heat of combustion of several fuels
Q51: 500 g of Al is heated to
Q53: 100.0 g of Cu (specific heat =
Q53: Coal contains an impurity that reacts with
Q54: How much heat is needed to raise
Q56: Consider the reaction:
Q59: The final temperature when 150 mL of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents