CO2 and H2 are allowed to react until an equilibrium is established as follows: 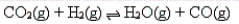 What will be the effect on the equilibrium of removing CO from the equilibrium mixture?
What will be the effect on the equilibrium of removing CO from the equilibrium mixture?
A) The equilibrium will favor the reactants side.
B) H2 concentration will double
C) CO and CO2 concentrations will double
D) H2 concentration will decrease and H2O concentration will increase
Correct Answer:
Verified
Q8: The proper assignment of oxidation numbers to
Q9: Whether a reaction is exothermic or endothermic
Q10: Which substance functions as a reducing
Q11: Catalysts are correctly characterized by each of
Q12: What is the change in oxidation
Q14: Which of the following general reaction types
Q15: For a system at chemical equilibrium:
A) Forward
Q16: Which of the following changes is
Q17: Which of the following reactions is
Q18: Which element is oxidized in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents