According to Le Chatelier's principle, which of the following changes will shift the position of the equilibrium to the left for the reaction 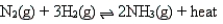
A) double the concentration of N2
B) decrease the concentration of NH3
C) increase the concentration of H2
D) triple the temperature
Correct Answer:
Verified
Q2: The minimum combined kinetic energy reactant particles
Q3: Combustion reactions are characterized by
A) oxygen always
Q4: Which of the following is the correct
Q5: The proper assignment of oxidation numbers to
Q6: Most reactions are carried out in liquid
Q8: The proper assignment of oxidation numbers to
Q9: Whether a reaction is exothermic or endothermic
Q10: Which substance functions as a reducing
Q11: Catalysts are correctly characterized by each of
Q12: What is the change in oxidation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents