The "setup" for the problem, "What is the mass, in grams, of 5.69 *1019 atoms of Xe?" which follows is correct, except numbers in the middle conversion factor have been replaced by the letters A and B. What are the numerical values of A and B, respectively? 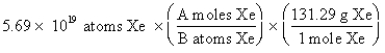
A) 1 and 6.02 * 1023
B) 1 and 131.29
C) 6.02 * 1023 and 1
D) 131.29 and 6.02 * 1023
Correct Answer:
Verified
Q12: Which of the following samples has the
Q13: Avogadro's number of sulfur (S) atoms would
Q14: A compound with the formula TeCln has
Q15: Which of following statements concerning theoretical yield,
Q16: Which of the following chemical equations
Q18: Which one of the following conversion
Q19: Potassium forms an oxide with the formula
Q20: A mole of a chemical substance represents
A)
Q21: The atomic masses of He and Be
Q22: In which of the following pairings of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents