Which of the following is the correct "setup" for the problem "How many grams of H2O will be produced from 2.1 moles of O2 and an excess of H2S?" according to the reaction 2H2S + 3O2  2H2O + 2SO2
2H2O + 2SO2
A) 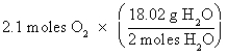
B) 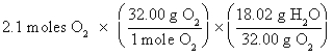
C) 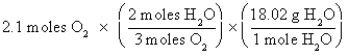
D) 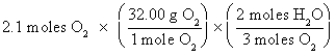
Correct Answer:
Verified
Q5: How many molecules of CO2 are present
Q6: To determine the formula mass of a
Q7: Which set of coefficients balances the
Q8: In the following reaction, how many grams
Q9: Which of the following is the correct
Q11: The formula mass of ammonium phosphite, (NH4)3PO3,
Q12: Which of the following samples has the
Q13: Avogadro's number of sulfur (S) atoms would
Q14: A compound with the formula TeCln has
Q15: Which of following statements concerning theoretical yield,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents