In the acid-base reaction: F-(aq) + HNO3(aq) 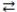 HF(aq) + NO3-(aq)
HF(aq) + NO3-(aq)
A) F- is the acid and its conjugate base is HF, and HNO3 is the base and its conjugate acid is NO3-(aq) .
B) HNO3 is the acid and its conjugate base is NO3-(aq) , and F- is the base and its conjugate acid is HF.
C) F- is the acid and its conjugate base is NO3-(aq) , and HNO3 is the base and its conjugate acid is HF.
D) HNO3 is the acid and its conjugate base is HF, and F- is the base and its conjugate acid is NO3-(aq) .
Correct Answer:
Verified
Q13: Which species can act as a Brønsted-Lowry
Q20: What is the expression for Kw, the
Q21: Ammonia,NH3,is an example of a _.
A)strong acid
B)strong
Q23: What is the pH of a cleaning
Q24: Which solution has the highest pH?
A)4.3 ×
Q25: Which acid has the strongest conjugate base?
A)Hydrogen
Q26: The [-OH] in a sample of egg
Q29: Which solution has the highest pH?
A)4.3 ×
Q32: Which acid is the strongest?
A)Hydrogen sulfate ion
Q40: The [H3O+] in a cabernet sauvignon wine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents