What is the molarity of an HNO3 solution if 24.1 mL of a 0.250 M Ba(OH) 2 solution are needed to titrate a 15.0 mL sample of the acid according to the equation below? Ba(OH) 2(aq) + 2 HNO3(aq) 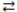 2 H2O(l) + Ba(NO3) 2(aq)
2 H2O(l) + Ba(NO3) 2(aq)
A) 0.402 M HNO3
B) 0.156 M HNO3
C) 0.311 M HNO3
D) 0.201 M HNO3
E) 0.803 M HNO3
Correct Answer:
Verified
Q20: What is the expression for Kw, the
Q22: Which buffer solution has the lowest pH
Q23: What is the pH of a cleaning
Q24: How many milliliters of 0.653 M
Q29: What is the pH of a peach
Q30: Which solution has the lowest pH?
A)1.3 ×
Q30: The pH of a lime is 1.90.
Q49: What is the conjugate base of the
Q55: Which salt forms a solution with a
Q59: What are the products of the acid-base
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents