When phosphoric acid (H3PO4) dissolves in water, the equilibrium shown below is established. If Ka = 7.5 × 10-3 for H3PO4, which statement concerning an aqueous solution of phosphoric acid is correct?
H3PO4 + H2O 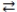 H3O+ + H2PO4-
H3O+ + H2PO4-
A) An aqueous solution of phosphoric acid contains mainly H3PO4 molecules.
B) An aqueous solution of phosphoric acid contains predominantly H3O+ and H2PO4- ions.
C) An aqueous solution of phosphoric acid contains equal amounts of H3O+ and H3PO4.
D) An aqueous solution of phosphoric acid contains a greater concentration of dissolved ions than it does neutral phosphoric acid molecules.
Correct Answer:
Verified
Q48: The pH of a 1.0 × 10-6
Q50: A sample of water from the Chesapeake
Q62: A solution in which [-OH] = 6.3
Q63: If two solutions differ in their [H3O+]
Q66: The pH of a 1.0 × 10-5
Q70: A solution in which [H3O+] = 7.4
Q71: The value of Kw = 1.0 ×
Q73: An aqueous solution of NaHCO3 is basic.
Q77: An aqueous solution of KCl has a
Q79: All compounds can be classified as either
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents