In the reaction: SO32-(aq)+ H2O(l) 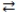 HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
Correct Answer:
Verified
Q74: In an acid-base reaction,a proton is transferred
Q75: The principal buffer in the blood is
Q76: An aqueous solution containing an equal number
Q78: If the pH of blood is lower
Q87: All salts where the anion is derived
Q89: OH- and NH4+ are both examples of
Q91: HOBr and CH3CH2CH2COOH are both examples of
Q92: Consider two acids,A and B. If acid
Q94: Consider two acids,A and B. If acid
Q97: A Brønsted-Lowry base must contain a lone
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents