The reactants are favored in the acid-base reaction: HF(aq)+ -OH(aq) 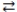 F-(aq)+ H2O(l).
F-(aq)+ H2O(l).
Correct Answer:
Verified
Q72: A solution containing a low concentration HCl
Q77: Most acids are weak acids.
Q82: Water is amphoteric; it can act as
Q84: For a solution labeled 0.15 M CH3COOH,the
Q85: The addition of an acid to water
Q86: HPO42- is amphoteric. The conjugate acid of
Q90: The reaction of a Brønsted-Lowry acid (HA)with
Q98: A compound that contains both a hydrogen
Q101: Each day,the stomach produces 2.0 L of
Q105: The stronger the acid,the (larger/smaller)_ the value
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents