The molecular art depicts the following reversible reaction at equilibrium: CO(g) + Cl2(g)  COCl2(g) . What can be inferred about the equilibrium constant, K, for this reaction?
COCl2(g) . What can be inferred about the equilibrium constant, K, for this reaction? 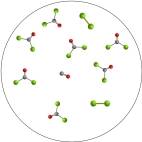
A) K < 1
B) K ~ 1
C) K > 1
D) K = 0
Correct Answer:
Verified
Q20: Catalysts accelerate a reaction by _.
A)lowering the
Q21: Which of the following is NOT
Q22: Consider the combustion reaction of propane:
Q23: Consider the reversible reaction at equilibrium: N2(g)+
Q24: Consider the reaction: C3H8(g)+ 5 O2(g)
Q26: Consider the reaction: PCl3(g)+ Cl2(g) 
Q27: Consider the reaction: N2(g)+ O2(g) 
Q28: A catalytic converter uses a catalyst to
Q29: Which of the following is always necessary
Q33: Walking at a brisk pace burns off
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents