Which of the following is NOT a reasonable assumption about the chemical reaction whose energy diagram is depicted below? 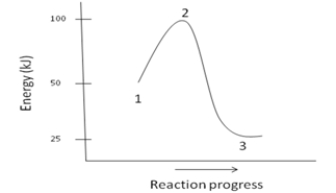
A) The activation energy for the reaction is 100kJ.
B) The reaction is exothermic.
C) H= -25kJ
D) The reaction is favorable.
Correct Answer:
Verified
Q3: A chemical reaction requires 31.39 kJ. How
Q16: Consider the reaction: C3H8(g)+ 5 O2(g)
Q17: A chemical reaction releases 55.2 kcal. How
Q19: Consider the reaction, 3 NO2(g)+ H2O(l)
Q20: Catalysts accelerate a reaction by _.
A)lowering the
Q22: Consider the combustion reaction of propane:
Q23: Consider the reversible reaction at equilibrium: N2(g)+
Q24: Consider the reaction: C3H8(g)+ 5 O2(g)
Q25: The molecular art depicts the following reversible
Q26: Consider the reaction: PCl3(g)+ Cl2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents