The hydrolysis of sucrose depicted below has K = 1.4 × 105. Which of the following is a reasonable assumption about this reaction once equilibrium is established? sucrose + H2O 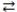 glucose + fructose
glucose + fructose
A) The equilibrium mixture contains equal amounts of sucrose, glucose, and fructose.
B) The equilibrium mixture contains mostly glucose and fructose.
C) The equilibrium mixture contains mostly sucrose.
D) The equilibrium shifts to the left due to the high value for K.
Correct Answer:
Verified
Q43: Exothermic reactions involve the formation of products
Q44: The difference in energy between the reactants
Q45: Which statement concerning the reversible reaction 2
Q47: Breaking a chemical bond requires energy.
Q53: One step in the metabolism of glucose
Q53: The energy of the reacting molecules affects
Q60: Changes in potential energy occur in chemical
Q67: Increasing the temperature of a reaction mixture
Q68: Chemical reactions are considered favorable if the
Q73: Activation energy is the minimum amount of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents