Consider the mixture of Cl2 and F2 in a closed container as illustrated below. What will the contents of the container look like if the molecules undergo the reaction: Cl2(g) + 3 F2(g) 2 ClF3(g) ? 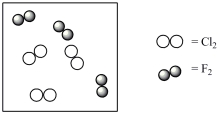
A) 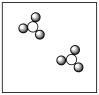
B) 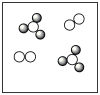
C) 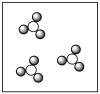
D) 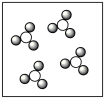
Correct Answer:
Verified
Q28: If a synthesis has four steps and
Q32: Consider the oxidation of sodium metal
Q35: In the balanced redox reaction: 2
Q35: Which quantity has the greatest mass?
A)2.0 mol
Q38: Sodium fluoride can be produced from
Q40: What are the two half reactions
Q46: Blimps are essentially very large helium filled
Q49: How many carbon atoms are in 77.28
Q52: What is the mass of 3.4 ×
Q56: The subscripts in chemical formulas are changed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents