A 100-g sample of the compound below contains less than 6.02 × 1023 molecules. 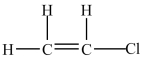
Correct Answer:
Verified
Q61: Consider the unbalanced chemical equation: NH3
Q61: The formula weight of a compound is
Q62: The reaction: Mg(s)+ 2 HBr(aq)
Q63: The actual yield of a product in
Q66: In the reaction: Ni2+(aq)+ Mg(s)
Q67: Consider the balanced reaction: 2 A
Q82: To multiply two numbers in scientific notation,multiply
Q88: One term in a balanced chemical equation
Q93: The molar mass of dibromomethane (CH2Br2)is larger
Q97: A chemical equation is an expression that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents