Which Atom(s)in the Structure Below Has(have)a Partial Negative Charge -)?
A)carbon
B)fluorine
C)hydrogen
D)nitrogen
E)nitrogen and Fluorine
Which atom(s) in the structure below has(have) a partial negative charge ( -) ? 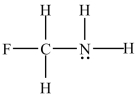
A) carbon
B) fluorine
C) hydrogen
D) nitrogen
E) nitrogen and fluorine
Correct Answer:
Verified
Q22: Which molecule's Lewis structure contains an atom
Q25: Estimate the bond angles around the sulfur
Q26: Which of the statements concerning compounds is
Q27: Which of the following is classified as
Q27: Which element may have more than eight
Q28: Which bond has the polarity incorrectly
Q29: What is the total number of bonding
Q32: What is the molecular shape around the
Q33: What is the molecular shape around the
Q35: How many lone pairs of electrons need
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents