The Lewis structure shown below is not a valid Lewis structure. What statement best describes the error in the structure? 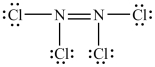
A) The nitrogen atoms violate the octet rule.
B) The chlorine atoms violate the octet rule.
C) The structure contains an incorrect number of valence electrons.
D) Chlorine atoms and nitrogen atoms do not typically form bonds with each other.
Correct Answer:
Verified
Q28: What is the correct chemical formula of
Q35: How many lone pairs of electrons need
Q37: Which Lewis structure is incorrect?
A) 
Q38: Which molecule or ion has only two
Q39: Which compound has the greatest number of
Q44: Phosphorus usually forms two covalent bonds in
Q52: Bonding is the joining of two atoms
Q55: A molecule is a discrete group of
Q71: Resonance structures for a substance differ only
Q77: Nonpolar molecules may contain polar bonds.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents