The Lewis structure of formaldehyde is shown below. Which statement concerning this structure is incorrect? 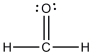
A) Two electrons are being shared between the carbon atom and the oxygen atom.
B) The oxygen atom has four valence electrons that are not being shared with another atom.
C) The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D) The hydrogen atoms have filled valence shells.
Correct Answer:
Verified
Q45: Atoms with three valence electrons generally form
Q59: Every atom must have an octet of
Q61: The formula for dinitrogen pentoxide is N5O2.
Q67: A bond formed between the elements hydrogen
Q69: A molecule that contains only one polar
Q76: A double bond consists of four electrons
Q78: The correct name for SF6 is sulfur
Q80: There can be a no more than
Q90: Dibromomethane (CH2Br2)is a nonpolar molecule.
Q98: A double bond is counted as two
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents